How can you be sure if a wound has harmful bacteria?
The answer - Bacterial autofluorescence for the detection of bacterial bioburden.
Kent Imaging Announces FDA 510(k) Clearance of SnapshotGLO.
ACTIONABLE & ACCURATE DIAGNOSTIC DATA
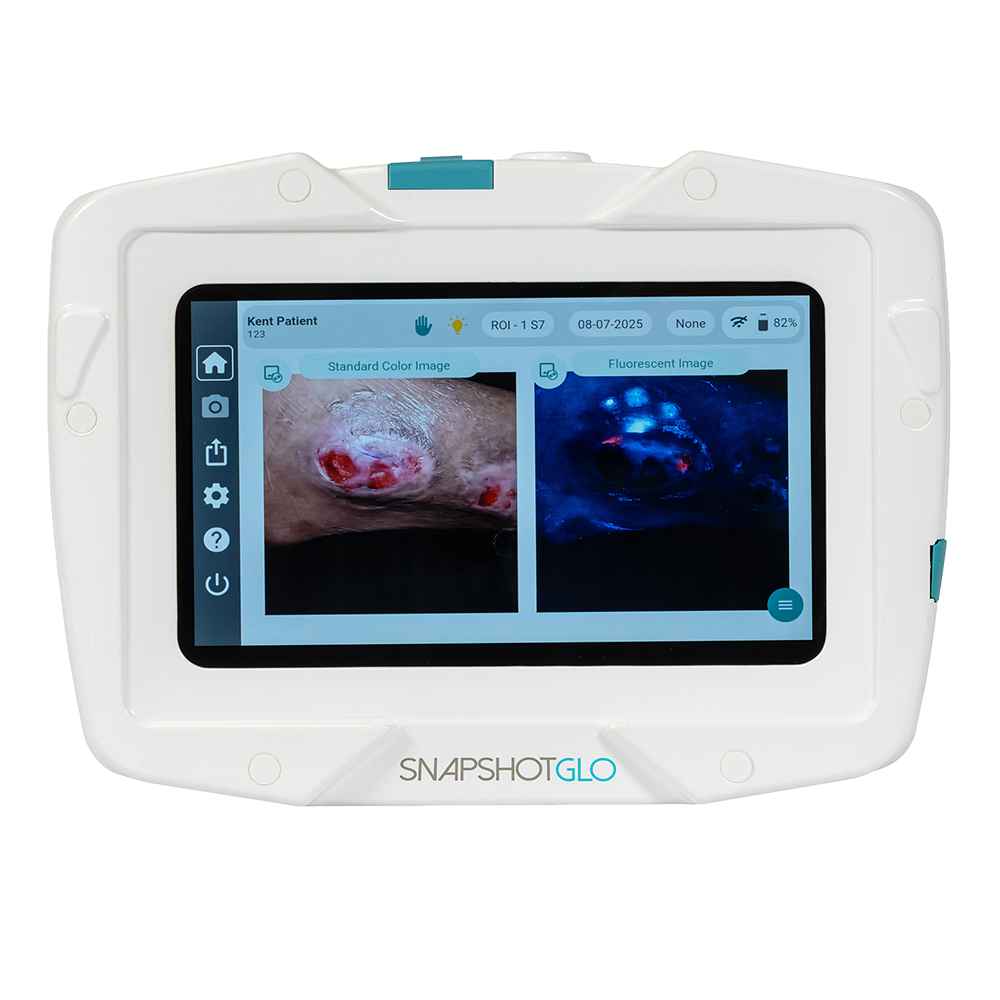
SnapshotGLO is a handheld, portable bacteria assessment device that measures wound bacterial bioburden, monitors wound healing and assists clinicians’ decision-making for first-line treatments.
Using safe, ultraviolet (UV) light, SnapshotGLO provides users with a visual map of elevated bacterial loads in a wound. The device can highlight areas of bacterial loads over 10(4) CFU/g*, aiding clinicians in providing timely, targeted interventions.
Bacteria contain fluorophores which become fluorescent when excited by UV light. As a non-contact, point-of-care device, UV light from SnapshotGLO is used to fluoresce bacteria on the wound surface, creating an image that indicates the location of bacterial load. The resulting image will show red and cyan fluorescence to highlight the most common types of bacteria found in infected wounds.
(*colony-forming units per gram)
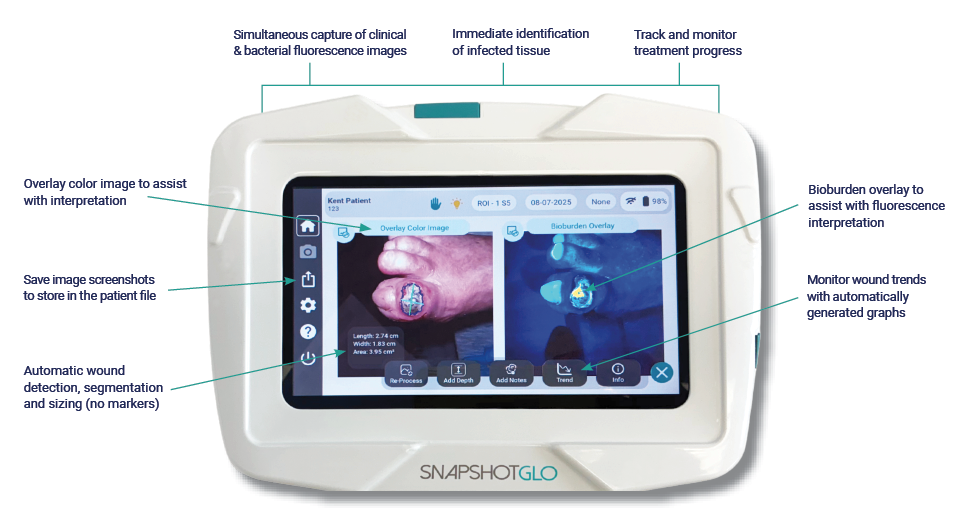
With a single image capture, and the UV light undiluted by a filter, SnapshotGLO identifies wound bacteria while simultaneously capturing a clinical image for wound auto detection, segmentation and sizing. No reagents or additional markers are required.
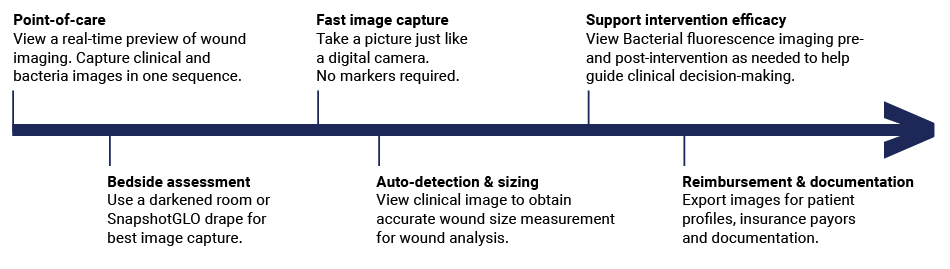
SnapshotGLO non-invasively assesses bacterial bioburden at the bedside, providing actionable information early in the care plan. This enables more targeted treatment, supports enhanced clinical decision-making, and helps to reduce the risk of improper antibiotic use—all leading to a lowered overall cost of care.
WHY DOES IDENTIFYING THESE FACTORS MATTER?
Delayed diagnosis can lead to deeper infection, sepsis, amputation, and death, particularly in vulnerable populations
Diagnosis of high bacterial loads can be difficult when patients present with multiple comorbidities
The presence & location of elevated bacterial loads can be challenging to identify through visual assessment.
Providers rely on clinical characteristics 98% of the time, on patient-reported symptoms 88% of the time, and on wound cultures 70% of the time.
What if there was a better way?
Cost-effective | Non-invasive | Immediate at the point of care
Easily integrate SnapshotGLO into your clinIcal workflow
Specifications
FDA 510(k) cleared
Protected by patent #11830190 Fluorescence-based detection of problematic cellular entities
weight
Device Weight - 1.27 Kg
Battery Charger - 0.54kg
Size
165mm x 228mm x92mm
(Height, Width, Depth)
SnapshotGLO comes with the following accessories
Battery Charger
2 USB sticks