Health Canada Approval for Kent Imaging’s tissue oxygenation device, SnapshotNIR KD205
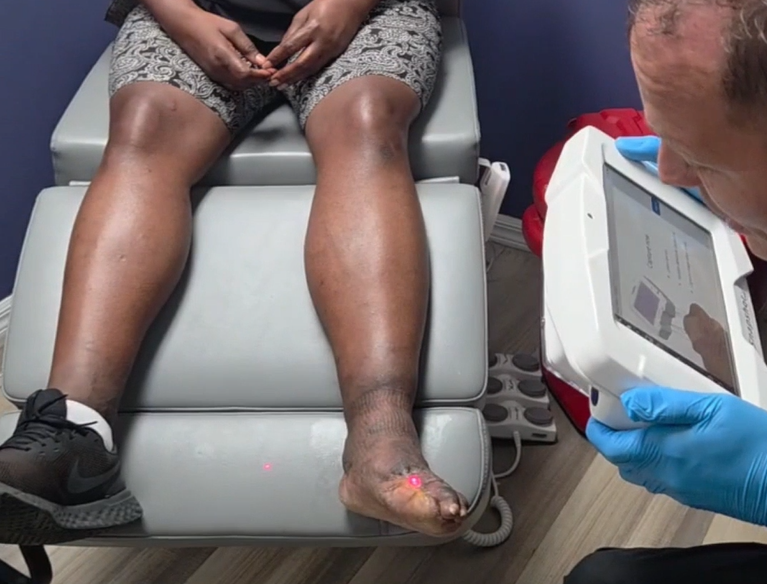
Kent Imaging Inc., a global leader in advanced diagnostic imaging solutions, is excited to announce that SnapshotNIR KD205, the company’s flagship near-infrared spectroscopy (NIRS) imaging device, has been licensed by Health Canada. FDA-cleared and CE-marked, SnapshotNIR KD205 delivers non-invasive, non-contact imaging to measure tissue oxygen saturation (StO2), oxyhemoglobin, deoxyhemoglobin, and total hemoglobin at the point of care, supporting clinicians in making informed decisions about tissue viability.
“With Health Canada approval, clinicians across Canada can access technology that delivers objective, real-time insights into tissue viability,” said Pierre Lemire, Chief Executive Officer of Kent Imaging. “SnapshotNIR KD205 offers exceptional versatility, whether it’s guiding surgical decisions in the operating room, supporting wound care management in outpatient clinics, or advancing research in academic and hospital settings. This adaptability ensures that healthcare professionals can rely on the same proven technology across multiple disciplines to improve patient outcomes.”
SnapshotNIR KD205 combines portability and ease of use with advanced technology designed to provide accurate, repeatable imaging results across nearly all Fitzpatrick Skin Types (FST) in soft tissue. By enhancing sensitivity to counter the light-scattering effect of melanin, the device automatically adjusts for varying skin tones, ensuring reliable measurements for a diverse patient population.
Kent Imaging continues to drive innovation forward across Canada and its home province of Alberta, advancing imaging technologies that support inclusive care, improve clinical decision-making, and enhance patient outcomes. Through ongoing research and development, Kent remains committed to delivering solutions that meet the evolving needs of clinicians nationwide.
To read more about the FDA 510(k) clearance of SnapshotNIR KD205, read this press release.
About Kent Imaging
Kent Imaging, located in Calgary, Alberta, Canada, is a leading innovator in advanced diagnostic imaging, developing, manufacturing, and marketing medical technology that supports real-time decision-making in wound care, vascular, and surgical subspecialties. Through patented imaging technologies, Kent continues to provide innovative solutions that aid healthcare systems nationally and internationally.
The Snapshot product suite now includes two powerful imaging devices: SnapshotNIR, a near-infrared tissue oxygenation imaging device initially cleared by the FDA and Health Canada in 2017 and CE-marked in 2025; and SnapshotGLO, a bacterial autofluorescence imaging device that received FDA 510(k) clearance in 2025. SnapshotNIR is supported by clinical evidence demonstrating its role in enhancing clinical decision-making, while SnapshotGLO provides real-time insight into wound bioburden presence and distribution. Together, the Snapshot product family strengthens clinicians’ ability to detect, direct, and protect—promoting treatment consistency, supporting improved workflow, and contributing to better patient care worldwide.