Looking for a better way to support payment for your
HBOT services?
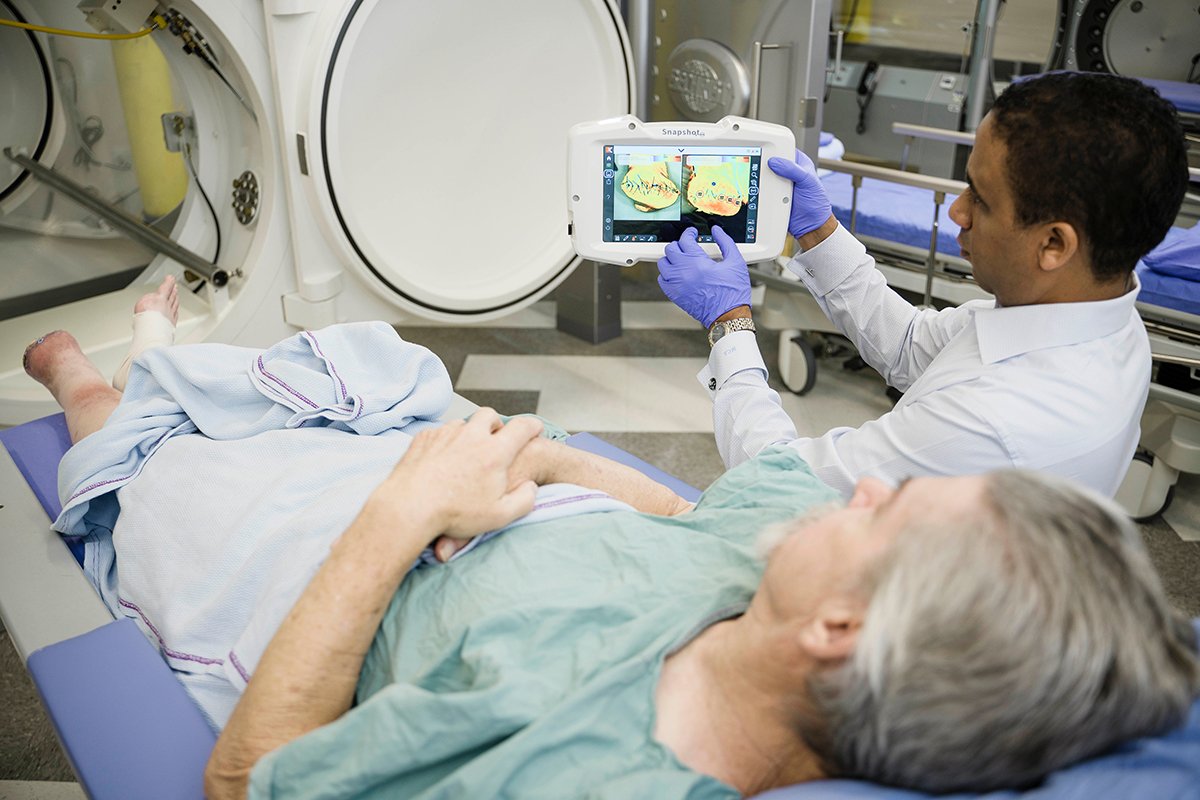
Qualify HBOT candidates, then track and document therapeutic efficacy.

SCREENING FOR PAD can
be challenging.
Early assessment with SnapshotNIR is fast, efficient and effective.
Support treatment decisions
and track healing ProgreSsion
Chronic wounds are a health problem with significant reductions in quality of life and can have devastating consequences such as limb amputations and premature death. SnapshotNIR is an imaging device that visualizes and maps tissue oxygen saturation in the capillary network.
Find out how SnapshotNIR supports treatment decisions, documents healing progression, and helps clinicians to positively impact patient outcomes, forging a path in diagnostic-driven wound care.
April 29, 2024
ADVANCING TISSUE ASSESSMENT
The latest model of SnapshotNIR is here! The device features upgrades that expand the application of the technology to include almost all skin tones on any location of the body.
May 9, 2024
TRANSFORMING DEBRIDEMENT
EZDebride (MDM Wound Ventures), an innovative debridement tool, and SnapshotNIR (Kent Imaging), a tissue oxygen saturation imaging device, are partnering to drive forward wound care standards and patient outcomes.
FULL WOUND CLOSURE OF STAGE IV SACRAL PRESSURE INJURY
Presented by: Danae Kissner: PA-C, CWS, CLT
Using NIRS imaging to confirm the improvement of StO2 allowed efficient wound care to completely heal the stage IV pressure injury in 35 weeks, less time than the national average healing time for this type of injury. The SnapshotNIR images demonstrated the transition from inadequate microcirculation to a proliferative healing environment throughout the patient’s treatment.
Initial visit of stage IV sacral pressure injury. Tissue oxygen saturation 59%.
Follow-up image 1-week post-intervention. Wound area reduced by 30%.
WOUND CARE
RECONSTRUCTIVE SURGERY







